Introduction: Multiple myeloma (MM) with extramedullary disease (EMD) is an aggressive disease that requires innovative treatment strategies.
Methods: The ongoing multinational, open-label phase 2 EMN19 trial (NCT04166565) enrolled patients (pts) with MM and EMD either as newly diagnosed MM (NDMM) or at first relapse (RMM). Pts received Daratumab (Dara) in combination with bortezomib (V), Cyclophosphamide (C), and Dexamethasone (D) (DaraVCD), either until disease progression or for a maximum of 36 months, along with autologous stem cell transplantation. Response to treatment was assessed using both the International Myeloma Working Group (IMWG) and the Impetus criteria for evaluating the EMD responses. Complete metabolic response (CMR) was defined as: Deauville scores 1, 2 or 3, with absence of FDG-avid bone marrow lesion(s), irrespective of a persistent mass on CT, or complete disappearance of EMD. Partial metabolic response as: Deauville score of 4 or 5 with decreased uptake compared to baseline and absence of structural progression development on CT or 50% reduction in EMD size. MRD assessment was performed locally at the time of CR or at an earlier time point, as per clinical practice. Pts without MRD assessment were included in the denominator when estimating the MRD(-) rate. Pts who had discontinued treatment by Cycle 4 were considered as non-responders (both in terms of hematologic and EMD response). Progression-Free Survival (PFS) was analyzed by the Kaplan-Meier method. Cox regression analysis with Firth's correction was used for the estimation of the hazard ratios (HRs) and 95% confidence intervals (CIs).
Results: A cohort of 40 pts (73% NDMM; 28% RMM) was enrolled. The mean age was 58 years, with 22 (55%) of the pts being male. At baseline, 33 (83%) of the pts were in stage ≤II of the International Staging System and 6 (15%) were classified as high-risk according to FISH (t(4;14), t(14;16) and/or del17p). Among the pts, 22 (55%) had extramedullary plasmacytomas (PCTs), 14 (35%) had paraosseous involvement, and 4 (10%) had both. The median number of PCTs per pt was 2. At the cut-off date with a median follow-up (FU) of 23 months, 17 (43%) pts were still on treatment, while 23 (58%) had discontinued treatment mainly due to disease progression (13, 33%), inadequate response at the end of cycle 3 (5, 13%) or death (3, 8%).
In the overall population, deepest responses were as follows: MRD(-) was achieved by 15 (38%) pts, ≥Complete response (CR) by 18 (45%) pts, ≥VGPR by 30 (75%) pts with an overall response rate (ORR) of 80% (32 pts). The median (range) time to MRD(-) was 19 months (4-33); 13 of the 15 MRD(-) pts were NDMM. Median PFS (24-months PFS rate) was 26 months (56%) in NDMM and 15 months (36%) in RMM.
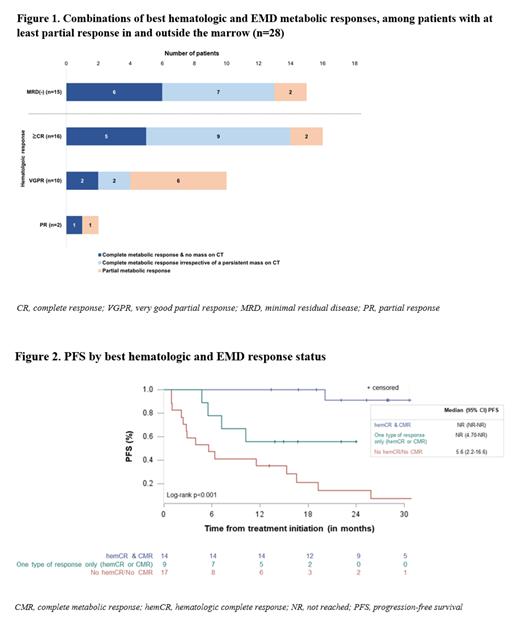
Overall, 28/40 (70%) pts had a concurrent hematologic and EMD response of partial response (PR) or better; the best hematologic responses achieved among those pts were: MRD(-) (15 pts), ≥CR (16 pts), VGPR (10 pts) and PR (2 pts). Additionally, 4/40 pts had a response of ≥PR (1 stringent CR, 1 CR and 2VGPR) without achieving an EMD response, while 8/40 pts had discontinued treatment by cycle 4 and did not have a hematologic or EMD response. The combinations of hematologic and EMD responses for 28 pts with concurrent hematologic and EMD response are shown in Figure 1. As seen in Figure 2, pts with concurrent hematologic CR (hemCR) and CMR had the best outcomes in terms of PFS, while having only one type of response (hematologic or EMD) was associated with inferior outcomes compared to having a response in and outside of marrow (p<0.001 overall and in NDMM). Overall, the respective HRs (95% CI) were: no response vs both types: 20.5 (3.5-119.8), p<0.001; one type vs both types: 8.3 (1.2-58.2), p=0.034. The median (range) time to first concurrent hemCR and CMR was 6 months (3-15); for pts achieving only one type of response (hemCR or CMR) the median (range) time to first response was 5 months (1-11).
Conclusions: After a median FU of 23 months of DaraVCD treatment, 33% of the pts have achieved both deep hematological responses with MRD(-) and CMR resulting in a median PFS of 20 months. However, having only one type of response (hemCR or CMR) is associated with an inferior outcome. These results, in terms of response, are comparable to those found in the LYRA study in which NDMM EMD pts are treated using a similar DaraVCD protocol. Even in this high-risk population of unmet need, long-term deep responses can be achieved with DaraVCD in and outside of marrow.
Disclosures
Beksac:Takeda: Speakers Bureau; Sanofi: Speakers Bureau; Menarini: Membership on an entity's Board of Directors or advisory committees; BMS: Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Gay:Roche: Other: Advisory board; Oncopeptides: Other: Advisory board; Bristol Myers Squibb/Celgene: Honoraria, Other: Advisory board; Sanofi: Honoraria, Other: Advisory board; Pfizer: Honoraria, Other: Advisory board; Takeda: Honoraria, Other: Advisory board; Janssen: Honoraria, Other: Advisory board; Amgen: Honoraria, Other: Advisory board; AbbVie: Honoraria, Other: Advisory board; GlaxoSmithKline: Honoraria, Other: Advisory board. Mina:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy; Pfizer: Honoraria; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Katodritou:Janssen Cilag, Amgen, Abbvie, Pfizer, GSK, Takeda, Sanofi, Karyopharm: Honoraria, Research Funding. Unal:Abbvie: Honoraria; Alexion: Honoraria; Novartis: Honoraria; Deva: Honoraria; Janssen: Honoraria. Cavo:GlaxoSmithKline: Honoraria; AbbVie: Consultancy, Honoraria; Amgen: Honoraria; Celgene/Bristol Myers Squibb: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; Sanofi: Consultancy, Honoraria; Takeda: Honoraria; Adaptive: Honoraria; Roche: Honoraria. Gokmen Sevindik:Takeda: Honoraria; MSD: Honoraria; BMS: Honoraria; Amgen: Honoraria; Jannsen: Honoraria. Manousou:Health Data Specialists: Current Employment. Sonneveld:Karyopharm: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding. Zamagni:Janssen: Honoraria; Bristol-Myers-Squibb: Honoraria; Takeda: Honoraria; Amgen: Honoraria. Terpos:Amgen: Honoraria, Other: Travel Expenses, Research Funding; GSK: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Sanofi: Honoraria, Other: Travel expenses, Research Funding; Pfizer: Honoraria; Takeda: Honoraria, Other: Travel expenses, Research Funding; EUSA Pharma: Honoraria, Other: Travel expenses; ASTRA/Zeneca: Honoraria, Other: Travel Expenses; BMS: Honoraria; Menarini/Stemline: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal